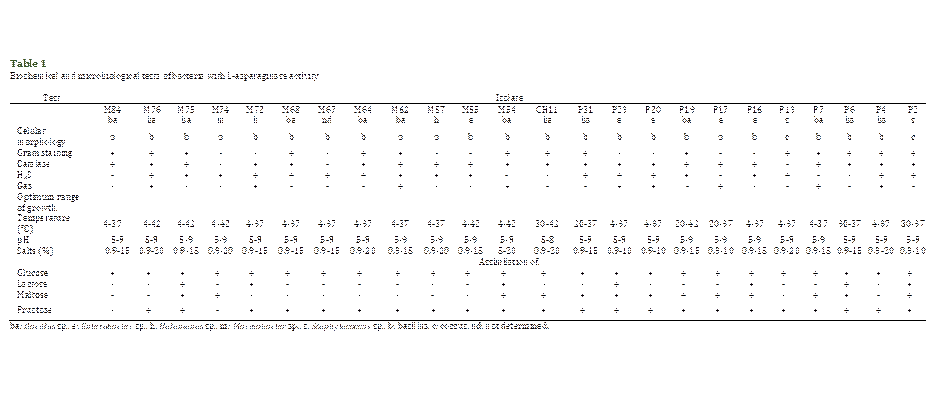Bacteria
producing L-asparaginase
isolated from Peruvian saline environments
Bacterias
productoras de L-asparaginasa aisladas de ambientes salinos peruanos
Jeanett Montes; Pamela
Elizabeth Canales*; Juan Carlos Flores-Santos; Stephy Saavedra; Abad
Hurtado-Gómez; Cynthia Esquerre-Huallpa; Amparo Iris Zavaleta
Laboratorio
de Biología Molecular, Facultad de Farmacia y Bioquímica, Universidad Nacional
Mayor de San Marcos, Lima, Perú.
*
Corresponding author: pamela.canales@unmsm.edu.pe
(P. E. Canales).
ORCID
ID of the authors
J.
Montes:  https://orcid.org/0000-0003-0981-8274 P.
E. Canales:
https://orcid.org/0000-0003-0981-8274 P.
E. Canales:  https://orcid.org/0000-0001-6019-8846
https://orcid.org/0000-0001-6019-8846
J.C.
Flores-Santos:  https://orcid.org/0000-0001-5632-7203 S.
Saavedra:
https://orcid.org/0000-0001-5632-7203 S.
Saavedra:  https://orcid.org/0000-0002-4867-7622
https://orcid.org/0000-0002-4867-7622
A.
Hurtado-Gómez:  https://orcid.org/0000-0002-4278-243X C.
Esquerre-Huallpa:
https://orcid.org/0000-0002-4278-243X C.
Esquerre-Huallpa:  https://orcid.org/0000-0002-2727-8884
https://orcid.org/0000-0002-2727-8884
A.
I. Zavaleta:  https://orcid.org/0000-0003-3844-7185
https://orcid.org/0000-0003-3844-7185
ABSTRACT
L-asparaginase (EC 3.5.1.1)
hydrolyzes L-asparagine in L-aspartic acid
and ammonia. Its efficiency is subject to its kinetics and specificity on the
substrate, characteristics that vary from one source to another. Thus,
microorganisms from saline environments constitute a phylogenetic and
metabolically heterogeneous group for the search of new enzymes. Therefore, the
objective of this study was the phenotypic and genotypic characterization of
bacteria with L-asparaginase
activity isolated from Maras, Pilluana and Chilca salterns. The 24 evaluated bacteria
were classified as 38% Gram-negative bacilli and 54% positive; and 8% Gram-positive
cocci. The majority grew in 5% salt water, pH 7.0 and 37 °C, all assimilated
glucose. Of the 24 bacteria that produced L-asparaginase in solid
medium, enzymatic activity was determined in submerged cultures by the Nessler
method in 14 of them. The CH11, M62, M64, M68, and P19 strains identified as Bacillus
sp., by partial sequencing of the 16S ribosomal gene, presented higher L-asparaginase
activity and instability due to the presence of proteases. Saline environments
bacteria are potential sources for the prospective production of L-asparaginase to
use them as a therapeutic agent and in the food industry.
Keywords: L-asparaginase; saline
environments; Bacillus; Enterobacter; Halomonas.
RESUMEN
L-asparaginasa (EC 3.5.1.1) hidroliza L-asparagina en ácido L-aspártico y amonio. Su
eficiencia está supeditada a su cinética y especificidad sobre el sustrato,
características que varían de una fuente a otra. Así, los microorganismos de
ambientes salinos constituyen un grupo filogenético y metabólicamente
heterogéneo para la búsqueda de nuevas enzimas. Por ello, el objetivo de este
estudio fue la caracterización fenotípica y genotípica de bacterias con
actividad L-asparaginasa aisladas de
las Salinas de Maras, Pilluana y Chilca. Las 24 bacterias evaluadas fueron
clasificadas como bacilos Gram negativos 38% y positivos (54%); y cocos Gram
positivos (8%). La mayoría creció en agua de sales 5%, pH 7,0 y 37 °C, todas
asimilaron glucosa. De las 24 bacterias productoras de L-asparaginasa en medio
sólido, se determinó la actividad enzimática en cultivos sumergidos por el
método de Nessler en 14 de ellas. Las cepas CH11, M62, M64, M68 y P19
identificadas como Bacillus sp., por secuenciamiento parcial de los
genes ribosómicos 16S, presentaron mayor actividad L-asparaginasa e
inestabilidad por presencia de proteasas. Estas bacterias de ambientes salinos
son fuentes potenciales para la producción prospectiva de L-asparaginasa de uso como
agente terapéutico y en la industria alimentaria.
Palabras clave: L-asparaginasa; ambientes
salinos; Bacillus; Enterobacter; Halomonas.
Recibido: 02-04-2021.
Aceptado:
10-05-2021.
L-asparaginase (EC
3.5.1.1) hydrolyzes L-asparagine in L-aspartic
acid and ammonium, it is present in bacteria, yeast, fungi, mammals, among
others. It is used as a chemotherapeutic agent in the treatment of acute
lymphoblastic leukemia. The L-ASNase (L-asparaginase)
formulations correspond to type II L-ASNases from Escherichia coli
and Erwinia chrysanthemi in their native or pegylated forms. However,
these enzymes have adverse effects such as hypersensitivity, pancreatitis, and
neurotoxicity (Shrivastava et al., 2016; Muneer et al., 2020). Therefore, there
is a pressing search for new sources of L-ASNase to
overcome existing limitations.
In this regard,
obtaining L-ASNase
from microorganisms that inhabit saline environments is an attractive
alternative due to the structural modifications of their proteins. Which allow
them to adapt to variable temperatures, pH and salinity that could give them
new properties and kinetic characteristics. These environments are widely
distributed in various geographical regions. They contain microorganisms
belonging to different taxonomic groups with wide metabolic diversity and
biotechnological potential (Ventosa et al., 1998; Oren, 2010; Corral et al.,
2019).
In general, the
enzymes of saline environments microorganisms present greater stability under extreme
conditions of pH and temperature. Which is why they are considered enzymes with
catalytic attractiveness under demanding reaction conditions, and they also
serve as a model to improve the genetic characteristics of existing ones.
Various authors have shown the great versatility of these organisms in the production
of hydrolases such as proteases, lipases, amylases, cellulases, inulinases,
pullulanases, pectinases and nucleases (Sánchez-Porro et al., 2003;
Delgado-García et al., 2012; Ruginescu et al., 2019).
Additionally,
the great demand for therapeutic enzymes with better kinetic and immunogenic
profiles such as L-ASNase puts enzymes from saline environments microorganisms
in the spotlight. Thus, L-ASNases isolated from Bacillus
sp. BCCS 034 (Ebrahiminezhad et al., 2011), Bacillus aryabhattai
(Shirazian et al., 2016), Halomonas elongata (Ghasemi et al., 2017), Marinobacter
sp., Paracoccus sp., Pseudomonas sp., Rhodococcus sp., Vibrio
sp., among others (Zolfaghar et al., 2019) indicate appropriate characteristics
for use in thermoprocesses in the food industry, and as a therapeutic and
diagnostic agent. In this regard, obtaining new bacterial sources that produce L-ASNase
allows us to have alternatives for the productive sectors that use this enzyme.
Consequently, the purpose of the study is to characterize L-ASNase-producing
bacteria from three Peruvian saline environments characterized by different
climates and altitudes.
Samples and
isolation
The study was
carried out using 24 bacteria isolated from the muds of Maras at 3380 m
(Cusco), Pilluana at 200 m (San Martín) and Chilca salterns at 3 m (Lima)
altitude, which belong to the strains collection of the Molecular Biology
Laboratory of the Faculty of Pharmacy and Biochemistry of the Universidad
Nacional Mayor de San Marcos. In the reactivation of the bacteria, a 5% Salt Water
(SW) medium supplemented with 0.5% yeast extract was used (Dyall-Smith, 2006).
The composition of 5% SW (g l-1) was NaCl 40.0, MgSO4.7H2O
5.83, MgCl2.6H2O 5.0, KCl 1.17, NaBr 0.13, CaCl2
0.083, NaHCO3 0.03. Solid SW medium was prepared by adding 15 g l-1
of agar.
Preselection of
bacteria with L-ASNase activity in solid medium
Bacteria were
grown on agar with modified M-9 medium according to Gulati et al. (1997),
supplemented with 5% SW, and incubated at 37 °C for 48 h. L-asparaginase
activity was detected by the formation of pink halos around the colonies.
Likewise, the modified Czapek Dox medium (Gulati et al., 1997) with the following
composition (g l-1) was prepared: Na2HPO4 6.0,
KH2PO4 2.0, NaCl 10.0, L-asparagine 10.0
(Sigma-Aldrich, MO, USA), ᴅ-glucose 2.0, MgSO4.7H2O
0.5, pH 6.0. The medium was supplemented with 2% agar and 0.007% bromothymol
blue (Sigma-Aldrich, MO, USA). L-asparaginase activity was detected by
the formation of a blue halo around the growth.
Physiological
characteristics
Gram-staining
was performed and the morphological characteristics of the selected colonies
were determined. In addition, the growth capacity of the bacteria was evaluated
at salt concentrations of 0.9, 5, 10, 15 and 20%, w/v; at pH of 5, 6, 7, 8 and
9, and at temperatures of 4, 20, 30, 37 and 42 °C. All tests were performed in medium
SW supplemented with 0.5% yeast extract and incubated at 37 °C for 48 h. In
addition, the ability to assimilate glucose, fructose, lactose, and maltose was
evaluated. For this last test, the isolates were cultured in 5% SW supplemented
with 0.1% yeast extract and the carbohydrate to be evaluated at 0.2% (w/v)
(Mata et al., 2002).
Biochemical
tests
For the
catalase test, the bacteria were cultured in 5% SW supplemented with 0.5% yeast
extract and incubated at 37 °C for 24 h. A small bacterial culture sample was
removed on a sterile slide and drops of 3% (v/v) hydrogen peroxide were added.
The formation of bubbles indicated the production of catalase (Miao et al.,
2014). For the production of H2S and gas, TSI agar supplemented with
5% SW was employed using the conditions described above. Blackening and bubble
formation or rupture of the culture medium indicated the production of H2S
and gas, respectively.
Amplification of 16S ribosomal gene
and molecular analysis
table
Bacteria DNA was
extracted using organic solvents as described by Flores-Fernandez et al.
(2019). The 16S rRNA gene was amplified using universal primers specific for
the Bacteria domain 16SBF 5'-AGAGTTTGATCATGGCTCAG-3' and 16SBR 5'-GGTTACCTTGTTACGACTT-3'.
The final volume of the amplification reaction was 25 µl, which contained 20 µM
of each primer, 200 µM of each dNTP, 50 mM KCl, 10 mM Tris/HCl, 0.1% (v/v) Triton
X-100, 1.5 mM MgCl2, 1.5 U Taq DNA polymerase and 50 ng of DNA. The
reaction conditions were 94 °C for 4 min, followed by 35 cycles at 94 °C for 45
s, 55 °C for 1 min and 72 °C for 45 s, with a final extension at 72 °C for 7
min. Then, the PCR products were separated by electrophoresis on 1% agarose
gel. DNA 1 kb ladder (Invitrogen, Waltham, USA) was used as a molecular weight
marker and was stained with ethidium bromide for the visualization of the
fragments.
The 16S
ribosomal gene was partially sequenced by Macrogen (Rockville, USA). The
nucleotide sequences obtained were compared to those deposited in the GenBank
database (https://www.ncbi.nlm.nih.gov/genbank/) using
the BlastN algorithm.
Assay in submerged culture of
bacteria producing L-ASNase
The bacteria
were cultured at 37 °C for 24 h, in Czapek Dox modified medium supplemented
with 0.009% phenol red (Sigma-Aldrich, MO, USA) or 0.007% bromothymol blue at
pH 5.6. Afterwards, the L-ASNase activity was considered
positive if the phenol red or bromothymol blue media changed to pink or blue,
respectively. Furthermore, the intensity of the colour was measured by
spectrophotometry at 560 and 620 nm; and the final pH of the cultures. The
positive strains with the highest L-ASNase activity were selected
for further studies.
Quantification of L-ASNase
activity by the Nessler method
The ammonium
released from the hydrolysis of L-asparagine was quantified using the
Nessler reagent as described by Imada et al. (1973) with the modifications
described below. The crude extract was obtained from the bacterial isolates
cultured in the medium proposed by Qeshmi et al. (2015). The reaction was
carried out in two stages. In the first, the reaction mixture containing 250 µl
of 50 mM Tris buffer (pH 8.6), 225 µl of ultrapure water, 25 µl of 189 mM L-asparagine
and 25 µl of the sample (crude extract) was incubated at 37 °C for 30 min.
Then, 25 µl of 1.5 mM trichloroacetic acid (TCA) was added to the mixture and centrifuged
at 2000 g for 2 min at 4 °C. In the second stage, 1.075 ml of ultrapure water
and 125 µl of Nessler's reagent (Merck, Darmstadt, Germany) were added to 50 µl
of the supernatant from the first stage. The absorbance at 436 nm was measured
in a multiwell plate reader (model Infinite M200PRO, brand TECAN). The amount
of ammonia was determined from the standard curve prepared with different
concentrations of ammonium sulfate. The L-ASNase activity
was expressed in U ml-1. One unit (U) of L-ASNase was
defined as the amount of enzyme that liberates 1 µmol of ammonia per min.
Quantitative
assay of proteolytic activity
The method
described by Coêlho et al. (2016) using azocasein as substrate (Sigma-Aldrich,
MO, USA) was employed with the following modifications. The crude enzyme
extract was obtained from the bacterial isolates grown in the medium proposed
by Qeshmi et al. (2015). The enzyme extract (500 µl) was mixed with 500 µl 0,6%
w/v azocasein (dissolved in 50 mM Tris-HCl, pH 8.5) and incubated for 30 min at
37 °C. Then, 500 µl of 10% TCA was added and centrifuged at 2000 g for 5 min.
Finally, 100 µl of the supernatant was mixed with 100 µl of 0.5 N NaOH and the
absorbance was read at 430 nm in the plate reader.
Preselection of bacteria producing L-ASNase
Preselected bacteria with L-asparaginase
activity were 24, of which 12, 11 and 1 came from Maras, Pilluana and Chilca salterns,
respectively. These bacteria were named M54, M55, M57, M62, M64, M67, M68, M72,
M74, M75, M76, M84, P2, P4, P6, P7, P13, P16, P17, P19, P20, P23, P31, and
CH11; where the first letter represents the name of the saline, followed by the
strain number.
Saline environments are
variable among them-selves in parameters such as salinity, ionic composition,
temperature, pH, and nutrients. Which mainly depend on the geographical
location, climate and season (Bell, 2012).
They harbour a great
diversity of microorganisms that, unlike their counterparts that live in
non-extreme conditions, possess enzymes capable of being active under adverse
conditions. Likewise, the enzymes of these organisms generally possess
particular structures as an adaptation strategy to extreme conditions (Ventosa
et al., 1998; Gomes & Steiner, 2004; Enache & Kamekura, 2010).
Consequently, these proteins could present new immunological or catalytic
properties important for the treatment of acute lymphoblastic leukemia (Ebrahiminezhad
et al., 2011), or the thermo-processing of foods (Batool et al., 2016).
The present study describes
bacteria with L-ASNase activity
isolated from saline environments located in different geographical, climatic
and altitude regions. Thus, Maras salterns generally support low temperatures,
high radiation; and they present saline concentrations influenced by
underground spring waters in the Andes (Maturrano et al., 2006). Pilluana
salterns, located in a tropical zone, contain sodium and chloride as the
majority ions (Valencia, 2000). And like Maras salterns, they are used as
sources of salt for food consumption. On the contrary, Chilca salterns, located
on the coast, contain a high concentration of sodium chloride and sulfate
(Chacón, 1980) and are traditionally used for medicinal baths. This diversity
of environments harbours bacteria that, due to their adaptation mechanisms,
could show enzymes with novel characteristics for biocatalysis at an industrial
level.
Although these three salterns
have been reported as sources of halophilic microorganisms with biotechnological
potential (Chávez-Hidalgo, 2010), the L-ASNase activity in bacteria from these
environments has not been explored. In this regard, for example, previous
studies in Iran described saline environments in which a varied number of
bacteria with L-ASNase activity
were isolated. Thus, Barati et al. (2016) reported 38 from diverse saline
environments. Ebrahiminezhad et al. (2011) described 11 from Maharloo salt lake;
and Zolfaghar et al. (2019) reported 29 bacteria between halophilic and
halotolerant from different saline areas of Iran.
Phenotypic and molecular
characterization of bacteria producing L-ASNase
Table 1 shows that 92% of
the bacteria were identified as bacilli, 38% Gram-negative, 54% Gram-positive;
and 8% Gram-positive cocci. Also, 88% were catalase-positive, 75% and 38%
produced H2S and gas, respectively. Besides, 54% grew in a temperature
range between 4 and 37 °C; and 21%, in the range of 4 to 42 °C. Regarding
saline tolerance, 29% showed the ability to grow from 0.9 to 20% of salts,
while 50% grew between 0.9 and 15%. Regarding the pH, 96% grew in the range of
5 to 9. In the assimilation of sugars, all the strains were able to use glucose
and 21% of the isolates used glucose, lactose, maltose, and fructose. Likewise,
the analysis of the partial nucleotide sequences of the 16S ribosomal genes
showed similarity with members of the genera Bacillus (13), Enterobacter
(5), Halomonas (2), Marinobacter (1), and Staphylococcus
(2) (Table 1).
Of the studied bacteria, 54%
belongs to the genus Bacillus, which groups together a wide collection
of bacilli ubiquitous in nature that use various carbon sources, with
predominance in saline environments, and industrially relevant (Canales et al.,
2014; Harwood et al., 2018; Ortiz & Sansinenea, 2019). It is evident in the
characteristics of the strains (Table 1) that they phenotypically and
metabolically correspond to a heterogeneous group, as has already been described
for this genus (Slepecky & Hemphill, 2006).
As observed in this work,
the production of L-ASNase is not exclusive to the Bacillus genus, for
example Barati et al. (2016) found Halomonas and Aidingimonas as
the largest producers of L-ASNase in their study of hypersaline environments
in Iran. Besides, Sudha et al. (2017) and Han et al. (2014) describe the
production of L-ASNase by Enterobacter
and Staphylococcus strains isolated from salty seafood. Likewise,
Zolfaghar et al. (2019) mention the genera Bacillus, Halomonas, Marinobacter,
among others as producers of L-ASNase. Microorganisms can inhabit environments
with different characteristics if they manage to adapt to their conditions (De
Wit & Bouvier, 2006).
Therefore, it is not
surprising that as studies elucidating the microbial diversity of different
geographic areas and samples increase (Muneer et al., 2020), new L-ASNase-producing
species will be described.
Selection of bacteria producing L-ASNase by submerged
culture
The methods for the
selection of microorganisms producing L-ASNase are based on the detection of ammonia
after the hydrolysis of L-asparagine by L-ASNase, which increases the
pH in the culture medium.
In this regard, in the
preselection of bacteria with L-ASNase activity, two types of semi-quantitative
tests were carried out using the indicator dyes phenol red or bromothymol blue to
better discriminate colour change and intensity (Gulati et al., 1997; Mahajan
et al., 2013). The L-ASNase production of Maras, Pilluana and Chilca salterns bacteria
in submerged culture was evi-denced by a colour change with two pH indicators
(Table 2). The cultures that presented the highest absorbance at 560 nm and 620
nm were selected, when phenol red and bromothymol blue were used, respectively (Truppo
& Turner, 2010). Thus, of the 24 strains evaluated, 14 were considered the
best producers of L-ASNase (Table 2).
L-ASNase and
proteolytic activities of bacterial strains
The quantification of the L-ASNase and
proteolytic activities of the bacteria selected from the submerged cultures is
presented in Figure 1, where the Bacillus sp. M68, M64, M62, P19 and
CH11 produced higher activity of both L-ASNase and proteases. However, of these
five, the P19 strain showed the lowest proteolytic activity (Figure 1b). It
should be noted, in the search for bacteria that produce L-ASNase, an
important factor to consider is the presence of extracellular proteases, which influences
the decrease in activity and stability per se of the L-ASNase secreted
into the culture medium.
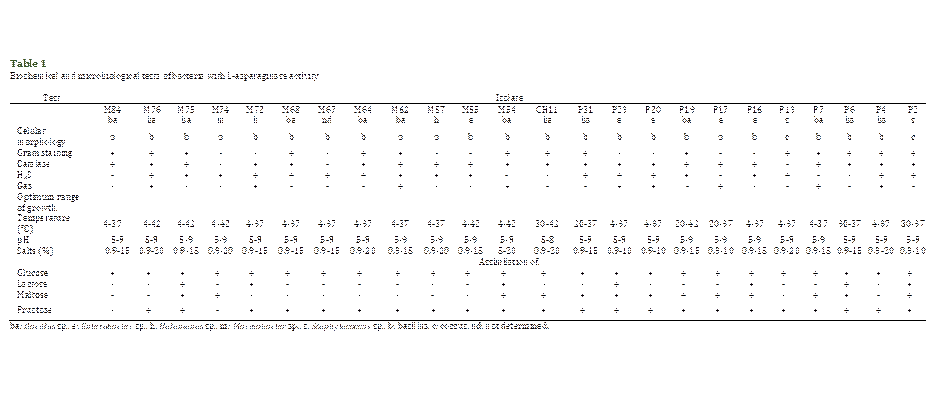
Table 2
Production of L-asparaginase in
submerged culture according to pH indicator
|
Strain code
|
bromothymol
blue
|
phenol red
|
|
pHfinal
|
colour change
|
OD620
|
pHfinal
|
colour change
|
OD560
|
|
M84
|
7.9
|
+
|
0.23
|
7.3
|
+
|
>3.00
|
|
M76
|
8.2
|
+
|
0.22
|
8.4
|
+
|
>3.00
|
|
M75
|
6.5
|
-
|
0.04
|
6.6
|
+
|
0.92
|
|
M74
|
8.1
|
+
|
0.16
|
8.0
|
+
|
>3.00
|
|
M72
|
8.5
|
+
|
0.31
|
8.0
|
+
|
>3.00
|
|
M68
|
7.6
|
+
|
0.14
|
7.9
|
+
|
>3.00
|
|
M67
|
7.9
|
+
|
0.13
|
8.1
|
+
|
>3.00
|
|
M64
|
8.1
|
+
|
0.22
|
8.1
|
+
|
2.74
|
|
M62
|
8.3
|
+
|
0.46
|
8.2
|
+
|
>3.00
|
|
M57
|
8.1
|
+
|
0.30
|
7.0
|
+
|
2.08
|
|
M55
|
7.0
|
-
|
0.07
|
6.5
|
+
|
0.92
|
|
M54
|
7.7
|
+
|
0.17
|
7.3
|
+
|
>3.00
|
|
P31
|
8.3
|
+
|
0.25
|
8.1
|
+
|
>3.00
|
|
P23
|
6.7
|
-
|
0.06
|
6.4
|
+
|
0.74
|
|
P20
|
6.7
|
-
|
0.05
|
6.5
|
+
|
0.62
|
|
P19
|
8.2
|
+
|
0.21
|
7.7
|
+
|
>3.00
|
|
P17
|
6.6
|
-
|
0.00
|
6.4
|
+
|
0.84
|
|
P16
|
6.7
|
-
|
0.00
|
6.3
|
+
|
0.52
|
|
P13
|
6.4
|
-
|
0.00
|
7.4
|
+
|
0.47
|
|
P7
|
6.8
|
-
|
0.06
|
7.5
|
+
|
0.56
|
|
P6
|
6.8
|
-
|
0.00
|
6.5
|
+
|
0.42
|
|
P4
|
7.9
|
+
|
0.18
|
7.3
|
+
|
>3.00
|
|
P2
|
6.5
|
-
|
0.00
|
7.3
|
+
|
0.37
|
|
CH11
|
8.2
|
+
|
0.19
|
8.1
|
+
|
>3.00
|
|
|
|
|
|
|
|
|
Initial pH of cultures: 5.6.
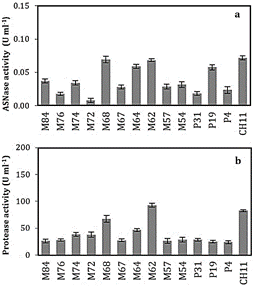
Figure
1.
L-asparaginase
(a) and protease (b) activities of bacterial strains.
In this regard, it has been
described that species of the genus Bacillus are protease producers
(Bhunia et al., 2012), as was found in the members of this genus M68, M64, M62,
P19 and CH11. These data are important to design cultivation strategies that
reduce or avoid the production of proteases. We suggest cultivation strategies
that include changing or adjusting the concentrations and the relationship of
the C/N sources. Additionally, include mixtures of protease inhibitors in the
purification processes. Likewise, molecular biology and genetic engineering
techniques may be used for gene cloning.
Microorganisms adapted to
saline environments are capable of resisting extreme conditions in more than
one environmental parameter. Thus, the bacteria in the present study resist a
wide range of pH, temperature and salt concentration. In addition, they all
assimilated glucose which would make them adaptable to various sources that
contain this monosaccharide. Likewise, since they come from saline
environments, their nutritional requirements are not demanding, which is
convenient in large-scale bioprocesses, and there is the possibility that they
have new immunological properties. It should be noted, of the 14 strains that
produce L-asparaginase in
liquid medium, five are potential sources for the prospective production of L-asparaginase,
and it is recommended to continue with their study and optimization of
production.
This work has been financed
with the agreement N° 169-FONDECYT-2017; and project VRIP-A17040034a. We thank
Nataly Allasi Canales for the English language review.
Barati, M., Faramarzi, M. A., Nafissi-Varcheh, N., Khoshayand, M. R.,
Tehrani, M. H. H., Vahidi, H., & Adrangi, S. 2016. L-asparaginase activity in cell lysates
and culture media of halophilic bacterial isolates. Iranian Journal of
Pharmaceutical Research, 15(3), 435-440.
Batool,
T., Makky, E. A., Jalal, M., & Yusoff, M. M. 2016. A comprehensive review
on L-asparaginase and its
applications. Applied Biochemistry and Biotechnology, 178(5), 900-923.
Bell, E. (Ed). 2012. Life at Extremes: Environments, Organisms and
Strategies for Survival (Vol. 1). Wallingford: CABI.
Bhunia, B., Basak, B., & Dey, A. 2012. A review on production of serine alkaline protease by Bacillus
spp. Journal of Biochemical Technology, 3(4), 448-457.
Canales, P. E., Chávez-Hidalgo, E. L., & Zavaleta, A. I. 2014.
Caracterización de bacterias halófilas productoras de amilasas
aisladas de las Salinas de San Blas en Junín. Revista Colombiana de
Biotecnología, 16(2), 150-157.
Coêlho,
D. F., Saturnino, T. P., Fernandes, F. F., Mazzola, P. G., Silveira, E., & Tambourgi,
E. B. 2016. Azocasein substrate for determination
of proteolytic activity: reexamining a traditional method using bromelain samples.
BioMed Research International,
2016, 1-6.
Corral, P., Amoozegar, M. A., & Ventosa, A. 2019. Halophiles
and their biomolecules: recent advances and future applications in biomedicine.
Mar Drugs, 18(1), 1-33.
Chacón,
G. 1980. Acción bactericida de la laguna minero-medicinal "Santa Cruz de
las Salinas" Chilca, Lima-Perú. Revista Peruana de Biología, 2(1),
8-51.
Chávez-Hidalgo,
E. L. 2010. Bacterias halófilas moderadas con actividad lipolítica aisladas de
las Salinas de Pilluana- San Martín. Thesis of Master. Universidad Nacional
Mayor de San Marcos, Lima, Peru.
De Wit, R., & Bouvier, T. 2006. ‘Everything is everywhere,
but, the environment selects’, what did Baas Becking and Beijerinck really say?
Environmental Microbiology, 8(4), 755-758.
Delgado-García, M., Valdivia-Urdiales, B., Aguilar-González, C. N.,
Contreras-Esquivel, J. C., & Rodríguez-Herrera, R. 2012. Halophilic hydrolases as a new tool for the biotechnological
industries. Journal of the Science of Food and Agriculture, 92(13),
2575-2580.
Dyall-Smith, M. 2006. The halohandbook. Melbourne: University of
Melbourne.
Ebrahiminezhad, A., RasouL-Amini, S., & Ghasemi, Y. 2011. L-asparaginase production by moderate
halophilic bacteria isolated from Maharloo Salt Lake. Indian Journal of Microbiology,
51(3), 307-311.
Enache, M., & Kamekura, M. 2010. Hydrolitic enzymes of
halophilic microorganisms and their economic values. Romanian Journal of
Biochemistry, 47(1): 47-59.
Flores-Fernandez, C. N., Chávez-Hidalgo, E., Santos, M., Zavaleta,
A.I., & Arahal, D.R. 2019. Molecular characterization of protease producing
Idiomarina species isolated from Peruvian saline environments. Microbiology
and Biotechnology Letters, 47(3), 401-411.
Ghasemi, A., Asad, S., Kabiri, M., & Dabirmanesh, B. 2017.
Cloning and characterization of Halomonas elongata L-asparaginase, a promising
chemotherapeutic agent. Applied Microbiology and Biotechnology, 101(19),
7227-7238.
Gomes, J., & Steiner, W. 2004. The biocatalytic potential of
extremophiles and extremozymes. Food Technology and Biotechnology, 42(4),
223-235.
Gulati, R., Saxena, R., & Gupta, R. 1997. A rapid plate assay for screening L-asparaginase producing microorganisms. Letters in Applied
Microbiology, 24(1), 23-26.
Han,
S., Jung, J., & Park, W. 2014. Biochemical characterization of L-asparaginase in NaCl-tolerant Staphylococcus
sp. OJ82 isolated from fermented seafood. Journal of Microbiology and
Biotechnology, 24(8), 1096-1104.
Harwood, C. R., Mouillon, J. M., Pohl, S., & Arnau, J. 2018.
Secondary metabolite production and the safety of industrially important
members of the Bacillus subtilis group. FEMS
Microbiology Reviews, 42(6), 721-738.
Imada, A., Igarasi, S., Nakahama, K., & Isono, M. 1973. Asparaginase and glutaminase activities of microorganisms. Journal
of General Microbiology, 76(1), 85-99.
Mahajan,
R. V., Saran, S., Saxena, R. K., & Srivastava, A. K. 2013. A rapid, efficient and sensitive plate assay for detection and
screening of L-asparaginase-producing
microorganisms. FEMS Microbiology Letters, 341(2), 122-126.
Mata, J. A., Martínez-Cánovas, J., Quesada, E., & Béjar, V.
2002. A detailed phenotypic characterisation
of the type strains of Halomonas species. Systematic and Applied
Microbiology, 25(3), 360-375.
Maturrano, L., Santos, F., Rossello-Mora, R., & Anton, J. 2006.
Microbial diversity in Maras salterns, a hypersaline environment in the
Peruvian Andes. Applied and Environmental Microbiology, 72(6),
3887-3895.
Miao, C., Jia, F., Wan, Y., Zhang, W., Lin, M., & Jin, W. 2014.
Halomonas huangheensis sp. nov., a moderately halophilic bacterium
isolated from a saline–alkali soil. International Journal of Systematic and
Evolutionary Microbiology, 64(3), 915-920.
Muneer,
F., Siddique, M. H., Azeem, F., Rasul, I., Muzammil, S., Zubair, M., Afzal, M.,
& Nadeem, H. 2020. Microbial L-asparaginase: purification,
characterization and applications. Archives of Microbiology, 202(5),
967-981.
Oren, A. 2010. Industrial and environmental applications of
halophilic microorganisms. Environmental Technology, 31(8-9), 825-834.
Ortiz, A., & Sansinenea, E. 2019. Chemical compounds produced
by Bacillus sp. factories and their role in nature. Mini Reviews in
Medicinal Chemistry, 19(5), 373-380.
Qeshmi, F.I., Rahimzadeh, M., Javadpour, S., & Poodat, M. 2015.
Intracellular L-Asparaginase
from Bacillus sp. PG02: purification, biochemical characterization and
evaluation of optimum pH and temperature. American Journal of
Biochemistry and Biotechnology, 12(1), 12-19.
Ruginescu, R., Purcarea, C., Dorador, C., Lavin, P., Cojoc, R.,
Neagu, S., Lucaci, I., & Enache, M. 2019. Exploring
the hydrolytic potential of cultured halophilic bacteria isolated from the
Atacama Desert. FEMS Microbiology Letters, 366(17), fnz224.
Sánchez‐Porro, C., Martin, S., Mellado, E., & Ventosa,
A. 2003. Diversity of moderately halophilic
bacteria producing extracellular hydrolytic enzymes. Journal of Applied
Microbiology, 94(2), 295-300.
Shirazian, P., Asad, S., & Amoozegar, M. A. 2016. The
potential of halophilic and halotolerant bacteria for the production of
antineoplastic enzymes: L-asparaginase and L-glutaminase. EXCLI Journal, 15, 268-279.
![]() https://orcid.org/0000-0003-0981-8274 P.
E. Canales:
https://orcid.org/0000-0003-0981-8274 P.
E. Canales: ![]() https://orcid.org/0000-0001-6019-8846
https://orcid.org/0000-0001-6019-8846![]() https://orcid.org/0000-0001-5632-7203 S.
Saavedra:
https://orcid.org/0000-0001-5632-7203 S.
Saavedra: ![]() https://orcid.org/0000-0002-4867-7622
https://orcid.org/0000-0002-4867-7622![]() https://orcid.org/0000-0002-4278-243X C.
Esquerre-Huallpa:
https://orcid.org/0000-0002-4278-243X C.
Esquerre-Huallpa: ![]() https://orcid.org/0000-0002-2727-8884
https://orcid.org/0000-0002-2727-8884![]() https://orcid.org/0000-0003-3844-7185
https://orcid.org/0000-0003-3844-7185